MDL 108736 (2023-01-09)-BV
With over 30 years of experience in scientific research and development, production, and sales of dental implant systems, Bioconcept provides a wide range of dental products, including implant systems, surgical instruments and tools, and digital solutions. We dedicate significant resources to continual innovation through our research and development team and world-class manufacturing processes. Our quality management system match or surpass leading global standards including FDA, CE, MDSAP, and other international certifications, providing you with world-class high-quality products.

 Material
MaterialOur implants are from Grade 4 (precision standard 6) titanium and dental prosthetics Grade 5 both imported from global leader ZAPP in Germany. Our surgical instruments and tools are made from highest medical grade stainless steel imported from global leader Sandvik in Sweden. Our commitment to only utilizing the highest quality materials across products serve as an important guarantee for long-term successful outcomes and efficacy of our products. Our materials acceptance standards far exceed those of ASTM, ISO, and other relevant standards, ensuring the highest reliability and consistency of all of our products.


 Mechanical Working - CNC
Mechanical Working - CNCAll products are milled using advanced CNC technology from Switzerland and Japan. Our cutting tools and components are sourced from leading Japanese and Swiss brands. Our processes and procedures are continually refined and optomized by our highly skilled technicians built on foundations of over 25 years experience milling world class medical devices and titanium implants.
 Cleaning
Cleaning Our implant cleaning processes involves multiple steps including high-pressure rinsing, steam cleaning, acid washing, ultrasonic washing, and vacuum cleaning. All of our cleansing agents are biologically safe and approved by the FDA. We have developed proprietary automatic cleaning equipment ensuring a highly consistent cleaning processes while also minimizing potential contaminations. Regular cleaning verification policies and procedures ensures consistent product quality and safety.
All water used in the cleaning process adopts secondary reverse osmosis and EDI (electro-deionization) technology with water quality exceeding purified water standards.


 Inspection
InspectionOur quality control processes consist of five primary inspection steps:
✔ Incoming Inspection
✔ Self-Inspection
✔ In-Process Inspection
✔ Finished Product Inspection
✔ Random Periodic Inspection
Advanced high-precision automated testing equipment and our professional inspection team ensures the use of only the highest-quality raw materials, accurate and precise continuous machining, and world class cleanliness and sterility standards.
Throughout the entire product manufacturing process, strict control and checks are conducted at every level to ensure the provision of high-quality products and clinical performance.
 Sanding
SandingAutomated sandblasting equipment creates a uniform and consistent micrometer-level medium roughness surface on the implant with increase roughness of Ra 1.5 ~ 2.5 ㎛.


 Etching
EtchingOur modern automated integrated acid etching and anodizing machines also contain self-cleaning functionality.
Through dual acid etching treatment, a dual morphological feature ranging from sub-micrometer to nanometer scale is formed on the surface, which better promotes a series of osseointegration biological behaviors such as cell adhesion, proliferation, secretion, and mineralization.
All batches are subject to scanning electron microscope inspection and analysis.
 Anodizing
AnodizingOur dental prosthetics are treated with anodization which improves the corrosion resistance and biological properties of our Grade 5 titanium. This treatment also creates different colors on our product surfaces making it easier for clinicians to identify and use.

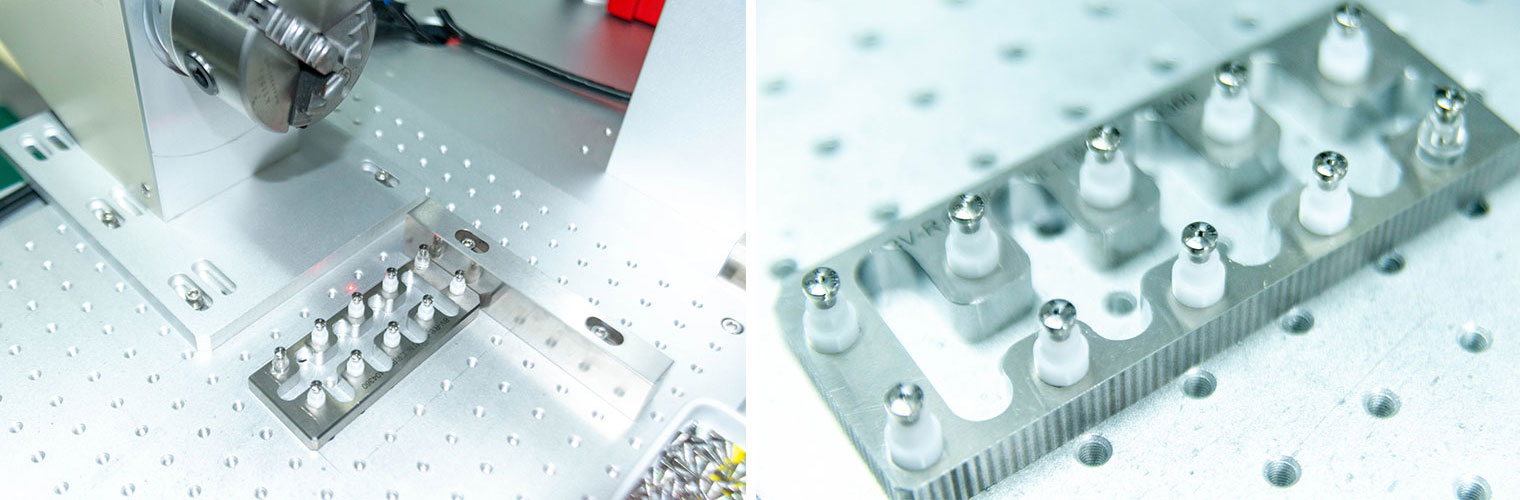
 Marking
MarkingFiber-optic laser marking equipment is employed to perform non-destructive printing on the product surface, creating functional scale lines, product specifications, and production information. This facilitates clinical identification and tracing, while ensuring corrosion resistance.
 Packing
PackingOur packaging is carried out in an ISO Class 8 (100000) cleanroom via automatic packaging lines. This provides highly efficient, repeatable, and controlled packaging.

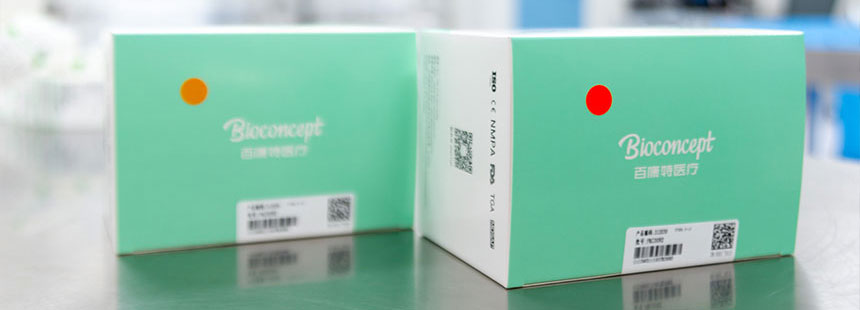
 Sterilization
SterilizationWe utilize Co60 γ-ray irradiation sterilization to ensure sterility of all Bioconcept products. Sterilization of products is confirmed through a sterilization indicator label which changes from orange to red upon exposure to gamma ray sterilization processes.
 Logistics
LogisticsOur products are stored in a world-class warehouse with controlled temperature and humidity. The receipt and shipment of goods are managed by our logistics team using leading ERP systems. Incoming inspection and multiple pre-shipment checks are conducted to ensure accurate and prompt delivery.

Bioconcept is a world-class medical manufacturing company that provides high-quality, high-precision medical products to the global market.
 Our implant system
Our implant system Our equipment and services
Our equipment and services Our quality system
Our quality system Our commitment
Our commitment Our pursuit
Our pursuitBioconcept promises to create the best clinical results for doctors and patients with high quality products and professional services!
